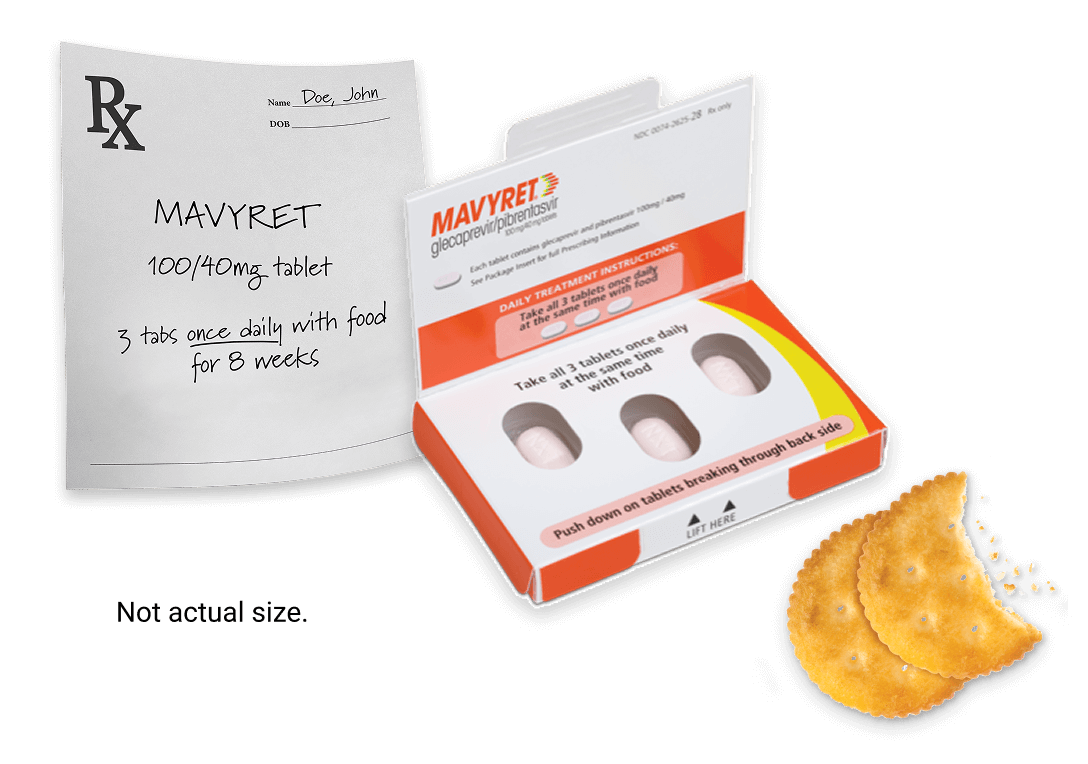
MAVYRET is 3 tablets taken once daily, at the same time, with food.
The Only Pangenotypic HCV Cure With Once-Daily Dosing, in Just 8 Weeks1,a,b
for TN, NC and CC Patients With HCV
aLiver or kidney transplant recipients are not eligible for an 8-week regimen. Refer to the full Prescribing Information
for additional dosing durations in other patient populations.
bMAVYRET is 3 tablets taken once daily, at the same time, with food.
AI-generated imagery. Not real patients.
MAVYRET is indicated for the treatment of adult and pediatric patients 3 years and older with acute or chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A). MAVYRET is indicated for the treatment of adult and pediatric patients 3 years and older with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both.1
Cure HCV in 8 Weeks With 1 Prescription Refill or Less for TN, NC and CC Patients1,a,b
Once-daily dosing in just 8 weeks
In all phase 3 clinical trials, patients were instructed to take MAVYRET with food without regard to fat or caloric intake.
- Each MAVYRET tablet contains 100 mg of glecaprevir and 40 mg of pibrentasvir (total daily dose: glecaprevir 300 mg and pibrentasvir 120 mg)
- MAVYRET is dispensed in a 4-week (monthly) carton. Each weekly carton contains 7 daily-dose wallets. Each monthly carton contains 4 weekly cartons
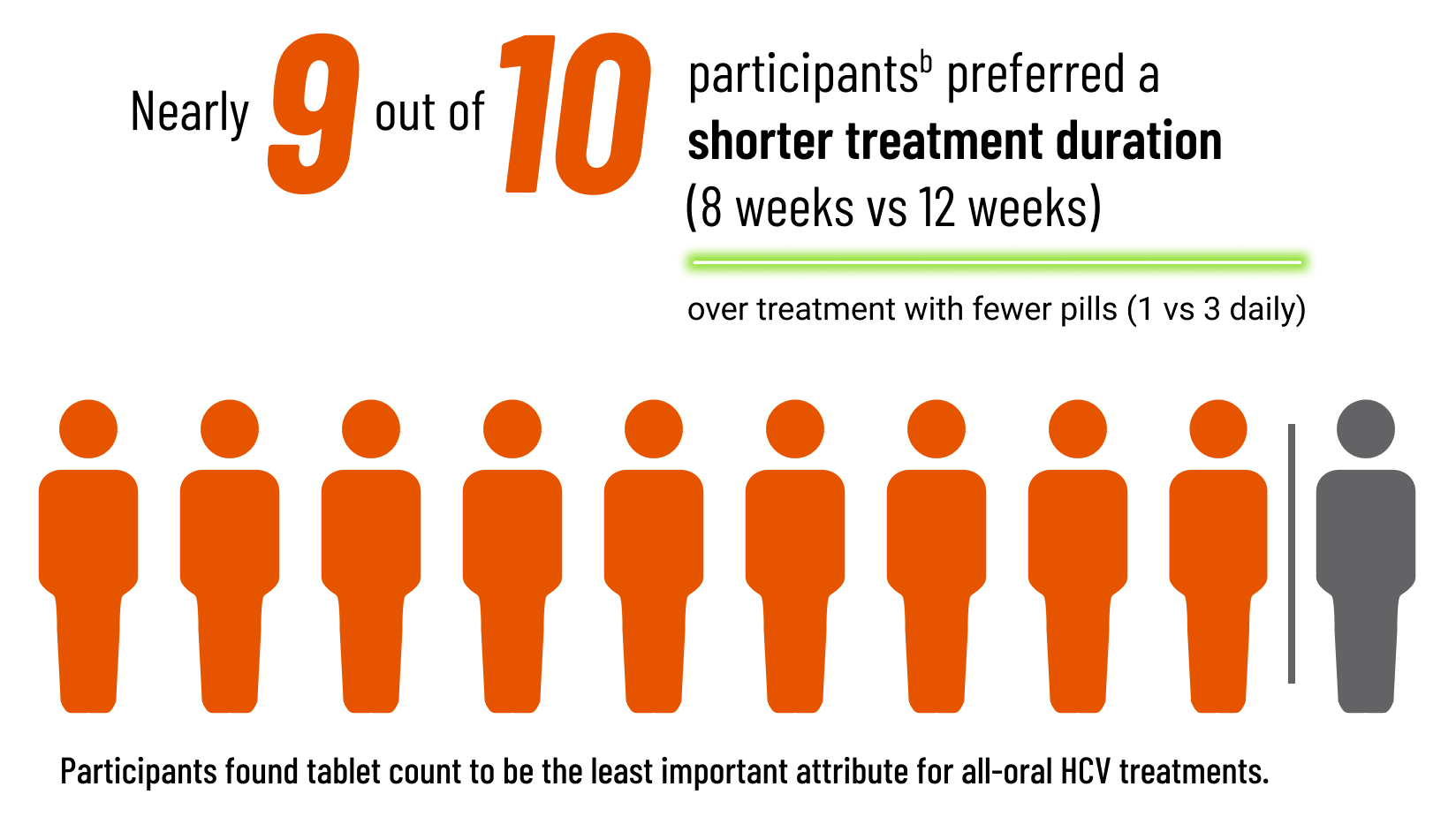
What could 8 weeks vs 12 weeks of treatment mean for patients with HCV?1
aNo refills needed when an 8-week dispense is allowed. One refill is needed when a 4-week dispense is required.
bLiver or kidney transplant recipients are not eligible for an 8-week regimen. Refer to the full Prescribing Information for additional dosing durations in other patient populations.
Dosing for Pediatric Patients 3 Years of Age or Older1
- The recommended dosage of MAVYRET in pediatric patients 3 to less than 12 years of age is based on weight, and MAVYRET oral pellets are recommended
- In pediatric patients 12 years of age and older, or in pediatric patients weighing at least 45 kg, the recommended dosage is 3 tablets taken at the same time once daily with food
Safety Considerations1
- MAVYRET is contraindicated in patients with moderate or severe hepatic impairment (Child-Pugh B or C) or those with any history of prior hepatic decompensation.
- MAVYRET is contraindicated with atazanavir or rifampin.
- Postmarketing cases of hepatic decompensation/failure, some fatal, have been reported in patients treated with HCV NS3/4A protease inhibitor–containing regimens, including MAVYRET. The median time to onset for MAVYRET was 27 days. The majority had moderate or severe hepatic impairment prior to initiating therapy, including some with compensated cirrhosis at baseline but with a prior decompensation event. Rare cases were reported in patients without cirrhosis or with compensated cirrhosis; many of these patients had evidence of portal hypertension. In patients with compensated cirrhosis or evidence of advanced liver disease, perform hepatic laboratory testing as clinically indicated; and monitor for signs and symptoms of hepatic decompensation such as the presence of jaundice, ascites, hepatic encephalopathy, and variceal hemorrhage. Discontinue MAVYRET in patients who develop evidence of hepatic decompensation/failure.
- Carbamazepine, efavirenz, and St. John’s Wort may significantly decrease plasma concentrations of glecaprevir and pibrentasvir, leading to reduced therapeutic effect of MAVYRET. The use of these agents with MAVYRET is not recommended.
Other Dosing and Administration Considerations1
If a dose is missed and it is:
- Less than 18 hours from the usual time that MAVYRET should have been taken–advise the patient to take the dose as soon as possible and then to take the next dose at the usual time
- More than 18 hours from the usual time that MAVYRET should have been taken–advise the patient not to take the missed dose and to take the next dose at the usual time
Additional Dosing Information1:
- HCV/HIV-1 coinfected patients and patients with any degree of renal impairment: follow the recommended dosage as detailed
- Liver or kidney transplant recipients:
- MAVYRET is recommended for 12 weeks in patients 3 years and older who are liver or kidney transplant recipients.
- A 16-week treatment duration is recommended in genotype 1–infected patients who are NS5A inhibitor-experienced without prior treatment with an NS3/4A PI or in genotype 3–infected patients who are PRS treatment-experienced.
MAVYRET Storage1
- Store at or below 30°C (86°F)

Treatment Duration: A Participant Preference Survey
Discover the ResultsHow MAVYRET Works
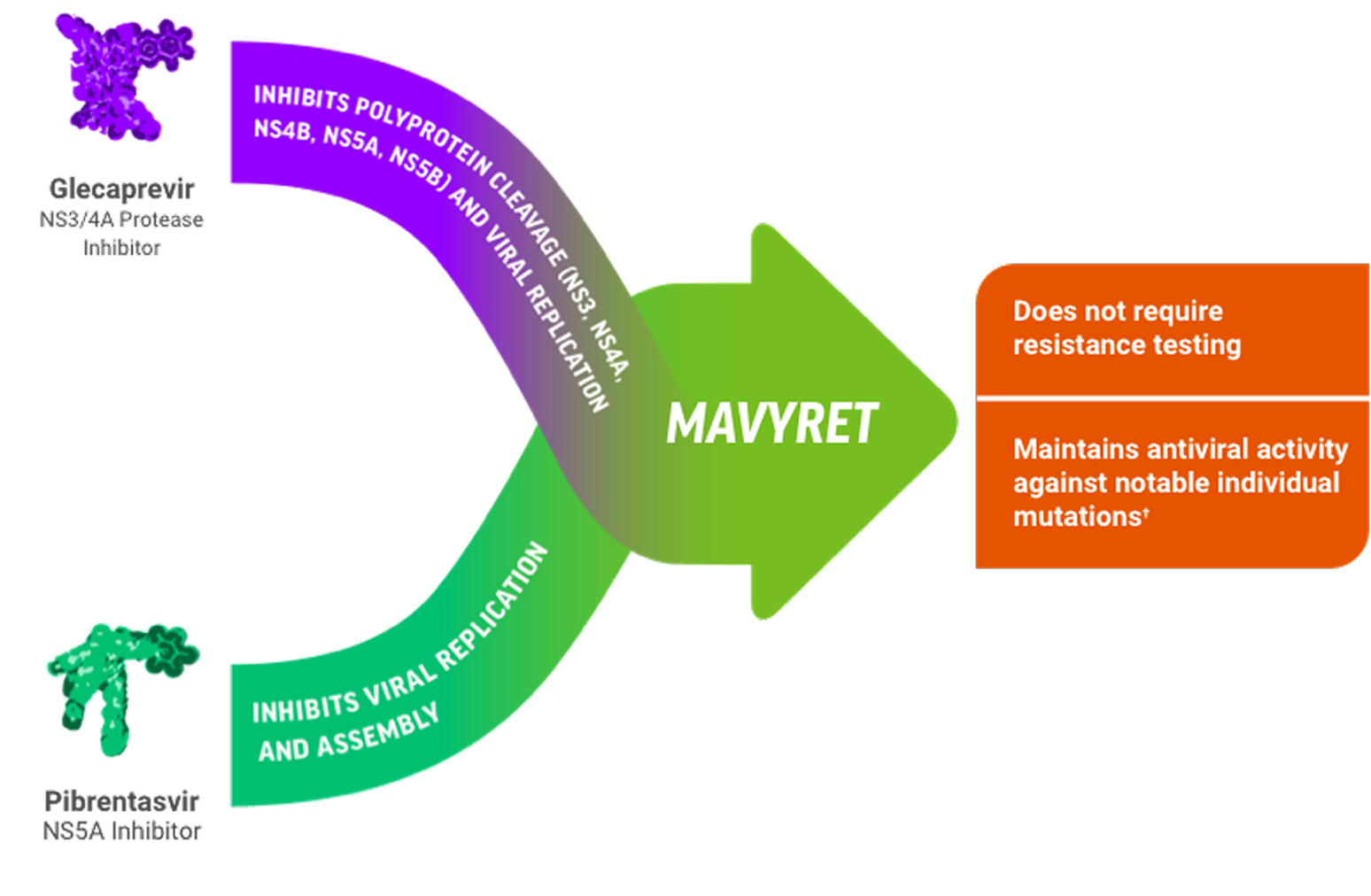
Potent Protease Inhibitor & NS5Ai Combinationc Designed to Stop Viral Replication1,2
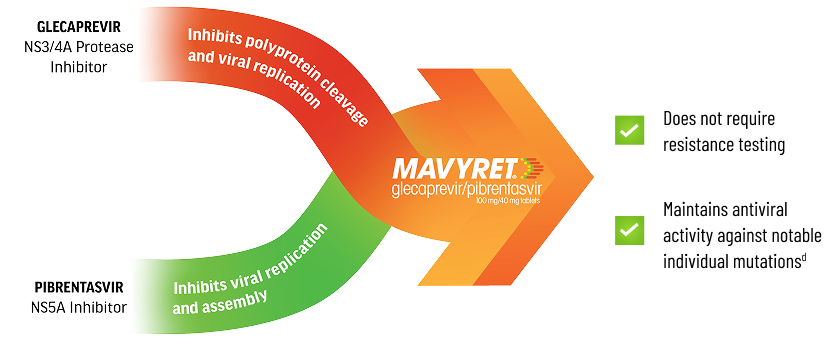
cPotency of antiviral activity is often measured using EC50 values determined by in vitro studies. The median EC50 values against the common subtypes of HCV GT 1-6 were 0.08-4.6 nM for glecaprevir and 0.5-15.6 pM for pibrentasvir.1
dRESISTANCE1
Glecaprevir: Individual amino acid substitutions associated with resistance to other HCV protease inhibitors at positions 36, 43, 54, 55, 56, 155, 166, or 170 in NS3 generally did not reduce susceptibility to glecaprevir. Individual substitutions at NS3 position D/Q168 had varying effects on glecaprevir susceptibility depending on HCV genotype/subtype and specific amino acid change, with the greatest reductions (>30-fold) observed in GT 1a (D168F/Y), 3a (Q168R), and 6a (D168A/G/H/V/Y). An NS3 Q80R substitution in GT 3a caused a 21-fold reduction in glecaprevir susceptibility, while Q80 substitutions in GT 1a and 1b (including GT 1a Q80K) did not reduce glecaprevir susceptibility.
Pibrentasvir: The majority of individual amino acid substitutions associated with resistance to other HCV NS5A inhibitors at positions 24, 28, 30, 31, 58, 92, or 93 in NS5A did not reduce susceptibility to pibrentasvir. Individual NS5A amino acid substitutions that reduced susceptibility to pibrentasvir included M28G or Q30D in a GT 1a replicon (244- and 94-fold, respectively), and P32-deletion in a GT 1b replicon (1036-fold). In a GT 3b replicon, the presence of naturally occurring polymorphisms K30 and M31 in NS5A reduced susceptibility to pibrentasvir by 24-fold relative to the activity of pibrentasvir in a GT 3a replicon. Introduction of an NS5A Y93H substitution into a GT 3b replicon further reduced susceptibility to pibrentasvir by 6336-fold.

Explore Efficacy Results and Treatment Outcomes
View Efficacy Data