OVERALL CURE RATES
POWERFUL CURE RATES IN JUST 8 WEEKS1,2*
In Treatment-Naïve Patients
| 98% | CURE RATE |
| (n=1218/1248, ITT) SVR12 Range: 95%-99% |
Based on an integrated, pooled analysis of GT 1-6 TN NC and CC adult patients across 8 clinical trials.

0.1% on-treatment virologic failure (n=1/1248)2
0.6% relapse (n=7/1226)2
Contraindicated in patients with moderate or severe hepatic impairment (Child-Pugh B or C) or those with any history of prior hepatic decompensation.1
*Liver or kidney transplant recipients are not eligible for an 8-week regimen.
Cure = sustained virologic response (SVR12); HCV RNA <LLOQ at 12 weeks after the end of treatment. Relapse = HCV RNA ≥LLOQ after end-of-treatment response among subjects who completed treatment.
AASLD and IDSA have not endorsed, and are not sponsors of, or otherwise affiliated with, MAVYRET or AbbVie Inc.


VIRAL SUPPRESSION ACROSS 8 WEEKS14
In non-cirrhotic patients completing 8 weeks of MAVYRET
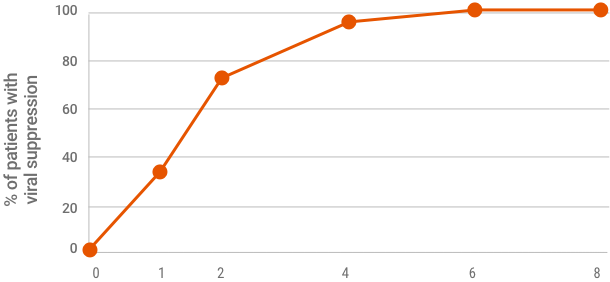
96%
OF PATIENTS WERE VIRALLY SUPPRESSED AT TREATMENT WEEK 4 (n=906/942)
Treatment Week
Viral suppression at treatment week 4 is not a clinical endpoint for cure.
Due to assay difference across studies, LLOQ was 25 IU/mL (Roche COBAS TaqMan® RT-PCR assay v. 2.0) or 15 IU/mL (Roche COBAS Ampliprep/TaqMan® RT-PCR assay v. 2.0).
METHODOLOGY14
Based on a post hoc exploratory pooled analysis from five phase 2/3 clinical trials of TN or PRS-TE adult patients with HCV GT 1-6 without cirrhosis who were treated with MAVYRET for 8 weeks. Patient characteristics were comparable across studies, and HCV RNA measures were collected at baseline, at treatment weeks 1, 2, 4, 5, and at the end of treatment. After excluding patients lost to follow-up or missing SVR12 data (n=13), or with on-treatment virologic failure (n=2), 950 patients were included in the study; 942 had week-4 viral suppression data. Viral suppression was defined as HCV RNA <LLOQ.
LIMITATIONS1,14
Product labeling for treatment duration should be followed regardless of HCV RNA levels at TW4. The included clinical studies were not powered to assess the impact of on-treatment viral suppression on SVR12. No conclusions should be made from this exploratory analysis.
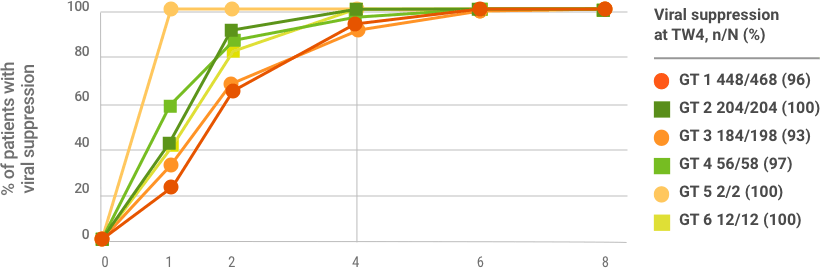
96%
OF PATIENTS WERE VIRALLY SUPPRESSED AT TREATMENT WEEK 4 (n=906/942)
Treatment Week
Viral suppression at treatment week 4 is not a clinical endpoint for cure.
Due to assay difference across studies, LLOQ was 25 IU/mL (Roche COBAS TaqMan® RT-PCR assay v. 2.0) or 15 IU/mL (Roche COBAS Ampliprep/TaqMan® RT-PCR assay v. 2.0).
METHODOLOGY14
Based on a post hoc exploratory pooled analysis from five phase 2/3 clinical trials of TN or PRS-TE adult patients with HCV GT 1-6 without cirrhosis who were treated with MAVYRET for 8 weeks. Patient characteristics were comparable across studies, and HCV RNA measures were collected at baseline, at treatment weeks 1, 2, 4, 5, and at the end of treatment. After excluding patients lost to follow-up or missing SVR12 data (n=13), or with on-treatment virologic failure (n=2), 950 patients were included in the study; 942 had week-4 viral suppression data. Viral suppression was defined as HCV RNA <LLOQ.
LIMITATIONS1,14
Product labeling for treatment duration should be followed regardless of HCV RNA levels at TW4. The included clinical studies were not powered to assess the impact of on-treatment viral suppression on SVR12. No conclusions should be made from this exploratory analysis.
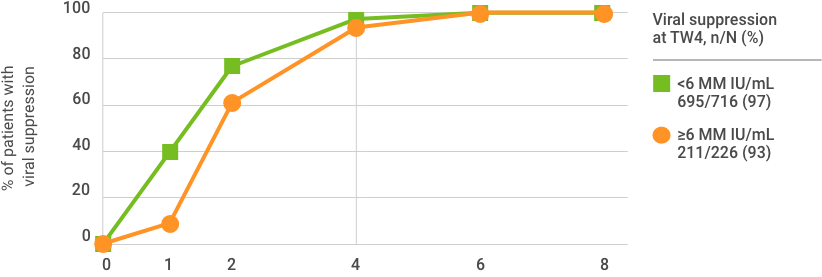
96%
OF PATIENTS WERE VIRALLY SUPPRESSED AT TREATMENT WEEK 4 (n=906/942)
Treatment Week
Viral suppression at treatment week 4 is not a clinical endpoint for cure.
Due to assay difference across studies, LLOQ was 25 IU/mL (Roche COBAS TaqMan® RT-PCR assay v. 2.0) or 15 IU/mL (Roche COBAS Ampliprep/TaqMan® RT-PCR assay v. 2.0).
METHODOLOGY14
Based on a post hoc exploratory pooled analysis from five phase 2/3 clinical trials of TN or PRS-TE adult patients with HCV GT 1-6 without cirrhosis who were treated with MAVYRET for 8 weeks. Patient characteristics were comparable across studies, and HCV RNA measures were collected at baseline, at treatment weeks 1, 2, 4, 5, and at the end of treatment. After excluding patients lost to follow-up or missing SVR12 data (n=13), or with on-treatment virologic failure (n=2), 950 patients were included in the study; 942 had week-4 viral suppression data. Viral suppression was defined as HCV RNA <LLOQ.
LIMITATIONS1,14
Product labeling for treatment duration should be followed regardless of HCV RNA levels at TW4. The included clinical studies were not powered to assess the impact of on-treatment viral suppression on SVR12. No conclusions should be made from this exploratory analysis.
CURE RATES IN PATIENTS WITH VARIED ADHERENCE15
Based on a retrospective, exploratory analysis of TN, CC or NC patients, excluding those with chronic kidney disease, from 10 phase 3 clinical trials15
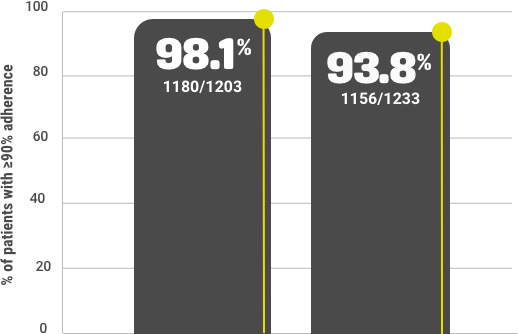
100%
CURE RATE WITH <90% ADHERENCE15
(mITT SVR12)
During weeks 0-4 (n=21/21) and weeks 5-8 (n=76/76)
99%
CURE RATE WITH ≥90% ADHERENCE15
(mITT SVR12)
During weeks 0-4 (n=1155/1162) and weeks 5-8 (n=1136/1143)
TELL YOUR PATIENTS THAT IT IS IMPORTANT NOT TO MISS OR SKIP DOSES OF MAVYRET DURING TREATMENT.
METHODOLOGY15
A post hoc, exploratory analysis evaluated adherence and SVR12 using pooled data from ten phase 3 clinical studies including TN HCV GT 1-6–infected adult patients without cirrhosis or with compensated cirrhosis. All patients received MAVYRET for 8 weeks. Adherence was calculated using available data on the percentage of pills taken relative to total number expected to be taken during each dispensation interval : interval No. 1 (weeks 0-4) and No. 2 (weeks 5-8), excluding any patient with missing pill count data for that interval.
LIMITATIONS15
The included clinical studies were not powered to assess differences between groups or the impact of adherence on SVR12; therefore, conclusions from this exploratory analysis should be drawn with caution. Patients with stage 4 or 5 chronic kidney disease, or with prior HCV treatment, were excluded. Patients with missing pill count data were excluded for each interval.
SELECT BASELINE CHARACTERISTICS15
Patient population characteristics of interest15
- 39% (n=510/1304) with history of injectable drug use
- 9% (n=111/1304) on stable opioid substitution therapy
- 33% (n=435/1304) currently using alcohol
- 27% (n=349/1304) with history of psychiatric disorder
- 27% (n=351/1304) on ≥5 concomitant medications
Patients with a history of psychiatric disorders were self-reported or defined as those with reported concomitant psychiatric medications. Patients using alcohol or with any history of injection drug use were self-reported.
Patient subgroups are not mutually exclusive.
PERCENT OF PATIENTS WITH ≥90% ADHERENCE
TELL YOUR PATIENTS THAT IT IS IMPORTANT NOT TO MISS OR SKIP DOSES OF MAVYRET DURING TREATMENT.
METHODOLOGY15
A post hoc, exploratory analysis evaluated adherence and SVR12 using pooled data from ten phase 3 clinical studies including TN HCV GT 1-6–infected adult patients without cirrhosis or with compensated cirrhosis. All patients received MAVYRET for 8 weeks. Adherence was calculated using available data on the percentage of pills taken relative to total number expected to be taken during each dispensation interval—interval No. 1 (weeks 0-4) and No. 2 (weeks 5-8), excluding any patient with missing pill count data for that interval.
LIMITATIONS15
The included clinical studies were not powered to assess differences between groups or the impact of adherence on SVR12; therefore, conclusions from this exploratory analysis should be drawn with caution. Patients with stage 4 or 5 chronic kidney disease, or with prior HCV treatment, were excluded. Patients with missing pill count data were excluded for each interval.
SELECT BASELINE CHARACTERISTICS15
Patient population characteristics of interest15
- 39% (n=510/1304) with history of injectable drug use
- 9% (n=111/1304) on stable opioid substitution therapy
- 33% (n=435/1304) currently using alcohol
- 27% (n=349/1304) with history of psychiatric disorder
- 27% (n=351/1304) on ≥5 concomitant medications
Patients with a history of psychiatric disorders were self-reported or defined as those with reported concomitant psychiatric medications. Patients using alcohol or with any history of injection drug use were self-reported.
Patient subgroups are not mutually exclusive.
